Abstract
Background:HDT with ASCT (HDT/ASCT) is an option as the upfront consolidation for poor-risk DLBCL. However, its role remains controversial and the more effective induction therapy with HDT/ASCT might improve the outcomes. To select a better induction therapy, JCOG conducted a randomized selection phase II study in previously untreated DLBCL patients (pts) with the high-intermediate (HI) or high (H) risk on age-adjusted International Prognostic Index (aaIPI) (UMIN000003823).
Methods: Previously untreated CD20+ DLBCL pts with aaIPI of HI and H were eligible. Other main inclusion criteria were: aged 20 to 65; stage II bulky, III or IV; ECOG performance status 0-2, at least 1 measurable lesion and preserved organ functions. Pts were randomized to receive induction therapy with six cycles of R-CHOP-14 (rituximab [R] 375 mg/m2, cyclophosphamide [CPA] 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 [max 2 mg], all IV on day (d) 1, and prednisone 100 mg PO on d 1-5, every 2 weeks) (Arm A) or 3 cycles of R-CHOP-14 followed by 3 cycles of CHASER (R 375 mg/m2 on d 1, CPA 1,200 mg/m2 on d 2, Ara-C 2 g/m2 on d 3-4, dexamethasone (DEX) 40 mg/body on d 2-4 and etoposide (ETP) 100 mg/m2 on d 2-4, every 3weeks) (Arm B). Randomization was balanced by institution and aaIPI score. Peripheral blood stem cells (PBSCs) with minimum of 2 x 106 CD34+ cells/kg were harvested after the 4th cycle of R-CHOP-14 or the 1st cycle of CHASER. Pts who achieved a complete response (CR) or partial response (PR) after induction therapy proceeded to HDT with LEED (melphalan 130 mg/m2 on d -1, CPA 60 mg/kg on d -4 and -3, ETP 500 mg/m2 on d -4 to -2 and DEX 40 mg/body on d -4 to -1) and ASCT on d 0. Radiation therapy was allowed to a residual disease after ASCT. The primary endpoint was 2-year (yr) progression-free survival (PFS). Secondary endpoints included overall response rates (ORR) and CR rates of both the induction phases and the entire treatment courses, 2-yr and 5-yr overall survival (OS) rates, 5-yr PFS rate, and safety. Assuming at least 65% of 2-yr PFS in both arms, sample size of 30 evaluable pts per arm would provide a correct selection probability of 80% in observing 10% better 2-yr PFS in one arm than in another. Estimating that up to 10% of pts would be ineligible, a sample size was set at 35 in each arm.
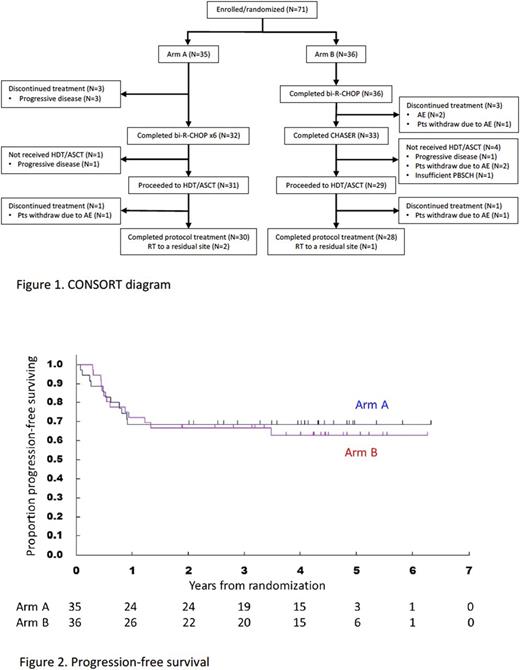
Results: Between June 2010 and February 2015, a total of 71 pts were randomized to Arm A (N=35) and Arm B (N=36). Baseline characteristics were (Arm A vs Arm B): male 18 (51%) vs 18 (50%); median (range) age 57 (23-64) vs 55.5 (30-65) yrs; aaIPI HI/H 24 (69%)/11 (31%) vs 28 (78%)/8 (22%). Thirty-two (91%) pts in Arm A and 33 (92%) in Arm B completed induction therapy, and 30 (86%) and 28 (78%) did study treatment per protocol, respectively (Figure 1). Progressive disease and discontinuation of the treatment due to toxicity were observed in 4 and 0 pts in Arm A and 1 and 5 in Arm B, respectively, before HDS/ASCT. In Arm A, 33 out of 35 pts yielded more than 2 x 106 CD34+ cells/kg and 34/36 pts in Arm B. With the median follow-up of 44.9 months (range: 4.2-75.9) among all pts, 2-yr PFS in Arms A and B were 68.6% (95% confidence interval [CI], 50.5-81.2%) and 66.7% (95%CI, 48.8-79.5%), respectively (Figure 2). OS at 2-yr were 74.3% (95%CI, 56.4-85.7%) in Arm A and 83.3% (95%CI, 66.6-92.1%) in Arm B. After induction therapy, ORR and CR rate were 88.6% and 62.9% in Arm A and 94.4% and 61.1% in Arm B, respectively, while 82.9% and 68.6 % in Arm A and 69.4% and 63.9% in Arm B, respectively, after entire therapy. Exploratory subgroup-analyses revealed a tendency of better PFS and OS in Arm B than those in Arm A in pts with aaIPI-H, bulky lesions (>=10cm) or stage non-IV. Of most common hematologic toxicities, grade (G) 3/4 neutropenia and G 3/4 thrombocytopenia were observed in 65.7% and 0 % respectively in induction phase of Arm A, 100% and 100% in induction phase of Arm B, and 97% and 100% in LEED (HDT/ASCT). G3 febrile neutropenia was observed in 17.1% in induction of Arm A, 55.6% in induction of Arm B and 44.8% in HDT/ASCT. In induction phase of Arm A and Arm B, 45.7% and 75.0% of pts had G_3 non-hematologic toxicities, respectively. Secondary malignancies were observed in 3 pts including: 1 prostatic cancer and 1 rectal cancer in Arm A and 1 lung cancer in Arm B.
Conclusions: R-CHOP-14 showed higher 2-yr PFS and less toxicity compared with R-CHOP-14/CHASER for HDT/ASCT in HI/H risk DLBCL pts by aaIPI. Thus, R-CHOP-14 was considered to be more promising induction regimen.
Yamamoto: AbbVie, Celgene, Ono Pharmaceutical, ARIAD Pharmaceuticals, Novartis, Takeda, Eisai, Solasia, MSD, Chugai, Gilead Sciences: Research Funding; Ono Pharmaceutical, Meiji Seika Pharma, Novartis, Otsuka Pharmaceutical, Mundipharma, Boehringer Ingelheim: Consultancy; ARIAD Pharmaceuticals/CMIC, Celgene, Novartis, Pfizer, Otsuka Pharmaceutical, Takeda, Kyowa Hakko Kirin, Bristol-Myers Squibb, Sumitomo Dainippon Pharma, Ono Pharmaceutical, Mundipharma, Chugai: Honoraria. Tobinai: GlaxoSmithKline: Research Funding; Kyowa Hakko Kirin: Honoraria, Research Funding; Ono Pharmaceutical: Honoraria, Research Funding; Eisai: Honoraria, Research Funding; Mundipharma: Honoraria, Research Funding; HUYA Bioscience: Honoraria; Daiichi Sankyo Co., Ltd: Consultancy, Honoraria; Servier: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Chugai: Honoraria, Research Funding; AbbVie: Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Zenyaku Kogyo: Honoraria. Uchida: Mundipharma K.K.: Research Funding; Janssen Pharmaceuticals: Honoraria. Shimada: Kyowa Hakko Kirin, Chugai, Eisai and Janssen: Honoraria. Fukuhara: Eisai, Janssen, Takeda, Ono and Zenyaku Kogyo: Honoraria; Nihon Ultmarc, Astellas, AbbVie, Alexionpharma, Bayer Yakuhin, Bristol-Myers Squibb, Baxalta, Celgene, Chugai, Daiichi-Sankyo, Toehringer Ingelheim, Eisai, GlaxoSmithKline, Janssen, Japan Blood Products Organization, Kyowa Hakko Kirin, Mitsubishi Tanabe, : Research Funding. Tokuhira: Ezai: Honoraria. Nagai: Janssen Pharmaceutical, Mundipharma, Celgene, Bayer Yakuhin, AbbVie, Takeda, Chugai, Kyowa Hakko Kirin and Eisai: Research Funding; Chugai, Mundipharma, Eisai, Takeda, Sanofi and Janssen: Honoraria. Kusumoto: Chugai: Honoraria, Other: GALLIUM and GOYA are sponsored by F. Hoffmann-La Roche Ltd. Third-party medical writing support, under the direction of Shigeru Kusumoto, was provided by Cheryl Wright of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd, Research Funding. Takamatsu: Takeda: Research Funding; Chugai: Research Funding; Kyowa Hakko Kirin: Research Funding; Ono: Research Funding; Astellas: Research Funding; TAIHO: Research Funding; Bristol-Myers Squibb: Research Funding; Pfizer: Research Funding; Taisho Toyama: Research Funding; Celgene: Honoraria. Nosaka: Celgene: Honoraria; Eisai: Honoraria. Maruyama: Chugai, Kyowa Hakko Kirin, Ono, Celgene, Janssen, Eisai, Mundipharma, Takeda: Honoraria; Dai-ichi Sankyo, Chugai, Kyowa Hakko Kirin, Ono, Celgene, Janssen, GSK, Eisai, Mundipharma, Takeda, AbbVie, MSD, Sanofi, Pfizer, Otsuka, Novartis, Solasia, Zenyaku: Research Funding. Hotta: SymBio Pharmaceuticals: Consultancy; SellSeed: Consultancy; Janssen: Honoraria; Meiji Seika Pharma: Honoraria; Zenyaku Kogyo: Honoraria. Tsukasaki: Chugai/Roche: Honoraria; Zenyaku Kogyo: Honoraria; HUYA: Honoraria; Mundypharma: Research Funding; Celgene: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Kyowa-Kirin: Honoraria; DaiichiSankyo: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal